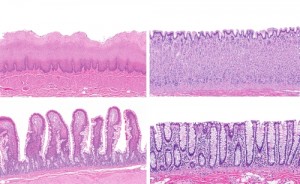
Great infographic image of the life cycle of MAP.
The part of this picture that should jump out at you is the statistic on the bottom left. citation # 3
92% !!!....... this is madness! This isn't new news people. So why doesn't anyone know about MAP and it's relation to Crohn's? I'm not talking about lay persons, but people in the profession of helping people get better who are living with this disease. They don't acknowledge it. The medical community flat out ignores the science and the 30 years or more of research that exists. People need to take charge of their life and health and start fighting to know something. Ask questions and demand an answer is where I'd start.
Click link below to view image larger from original source: