I get so excited when I read about this possible treatment, RHB-104, for Crohn's! I've been watching this drug for over a year now. This is a treatment that if approved by the FDA, I will be jumping on it as soon as it becomes available. No doubt! This is the kind of treatment we need. Everything else just treats symptoms.
So, why is this so exciting? If you read current research about what causes Crohn's disease, you will see that researchers are leaning away from considering Crohn's disease an autoimmune condition. They have found that there is an underlying cause for the immune response to activate. Crohn's is said to be caused by a combo of 3 factors - Immune system abnormalities, environmental influences and bacteria. I just wiki'd Crohn's disease and this is what is said about what is believed to be the cause - "Crohn's disease is caused by interactions between environmental, immunological and bacterial factors in genetically susceptible individuals.[4][5][6] This results in a chronic inflammatory disorder, in which the body's immune system attacks the gastrointestinal tract possibly directed at microbial antigens.[5][7] While Crohn's is an immune related disease, it does not appear to be an autoimmune disease (in that the immune system is not being triggered by the body itself)."
What this means is that people that have Crohn's disease do not have the immune system capabilities to destroy a form of bacteria that have been introduced into the body at some point in time. As research has indicated, a large number of people with Crohn's disease happen to have the MAP bacteria in their body somewhere. The exact location of the bacteria is not known at this time. Who cares where it is, as long as a treatment is available that will destroy it is my opinion.
See why this makes me super hopeful? If Crohn's is caused by this MAP bacteria & if this new combination antibiotic eradicates the MAP bacteria from people with Crohn's disease, this could be the answer to the Crohn's disease epidemic! Can someone say CURE :)
Wouldn't that be glorious?
Clinical trial for a new antibiotic therapy for Crohn’s treatment started:
Crohn’s disease is an inflammatory bowel disease which may affect gastrointestinal tract from mouth to anus, and has a wide variety of symptoms. Mycobacterium avium subspecies paratuberculosis (MAP) is believed to be associated with Cronh’s disease. Professor Dr. Saleh Naser, UFC College of Medicine, believes that MAP is the cause of the disease. He said, "Crohn's disease affects more than 750,000 Americans, yet traditional treatments only address the symptoms of inflammation and not the cause." He further added, "I have seen case studies where patients' lives have been restored following treatment, which removes MAP. I have high hopes that this clinical trial may lead to finding a cure." Dr. Saleh Naser will soon conduct clinical trials on a new antibiotic therapy acquired by RedHill Biopharma for curing the disease. 240 participants will be recruited for this double blind clinical trial.
http://health-beauty-2468.blogspot.com/2013/09/clinical-trial-for-new-antibiotic.html
RedHill Biopharma to initiate phase III trial of RHB 104 in Crohn's disease patients
RedHill Biopharma to initiate phase III trial of RHB 104 in Crohn's disease patients
UCF College of Medicine professor Dr. Saleh Naser soon will participate in a clinical trial to test whether a new antibiotic therapy acquired by RedHill Biopharma can be used to treat Crohn's disease patients.
The FDA-approved phase III trial is expected to commence within weeks by RedHill Biopharma, which licensed Naser's DNA technology for detecting Mycobacterium aviumsubspecies paratuberculosis, known as MAP. It is believed to be associated with Crohn's disease. RedHill Biopharma developed the anti-MAP antibiotic regimen known as RHB 104. Crohn's disease is a chronic inflammatory disease of the gastrointestinal tract characterized by cramping and diarrhea.
Naser developed and patented a way to detect MAP from milk, blood and tissue clinical samples. The bacterium is known to cause inflammation in the intestines of cows. It is also linked to Crohn's disease, although its role has been debated for more than a century. Naser believes MAP is an underlying cause of the disease.
"Crohn's disease affects more than 750,000 Americans, yet traditional treatments only address the symptoms of inflammation and not the cause," Naser said. "I have seen case studies where patients' lives have been restored following treatment, which removes MAP. I have high hopes that this clinical trial may lead to finding a cure."
RedHill will be enrolling 240 subjects from the United States, Canada and Israel in this double blind clinical trial in which blood and intestinal biopsy specimens from Crohn's patients will be tested for MAP before, during and following the one-year treatment with the antibiotic RHB 104.
"Since we acquired the license to Dr. Saleh Naser's MAP detection technique in 2011, we have had an excellent collaboration with UCF," said RedHill's CEO Dror Ben-Asher. "The UCF team of researchers- is at the forefront of global academic research on MAP and its detection."
Naser is looking forward to the trial and hopes this will end the academic debate regarding MAP and Crohn's disease.
"I am ecstatic to be part of a team, which will help determine whether or not MAP is associated with Crohn's disease; certainly a final answer to a one hundred-year old controversy," Naser said.
Source: University of Central Florida
http://www.news-medical.net/news/20130918/RedHill-Biopharma-to-initiate-phase-III-trial-of-RHB-104-in-Crohns-disease-patients.aspx
RedHill Readying Phase III Trials In Crohn's
With a PDUFA target date of Feb. 3, 2014 for its migraine drug, RHB-103, and NDA filing planned for the first quarter of 2014 for the anti-emetic drug, RHB-102, RedHill Biopharma (RDHL) is now focusing its efforts on its flagship programs: RHB-104 for Crohn's disease and RHB-105 for Helicobacter pylori (H. pylori).
RedHill is preparing to begin a potential groundbreaking Phase III study in the current quarter in North America and Israel (the MAP U.S. Study) of Crohn's disease, using a novel patent-protected formulation that combines three antibiotic ingredients in a single capsule, and is planning and preparing a parallel Phase III study in Europe (the MAP Europe Study).
"Following the discovery of the link between H. pylori bacterium and peptic ulcers, there is a growing body of evidence supporting the proposition that Crohn's disease and other so-called autoimmune diseases, such as multiple sclerosis, are linked to infections," chief business officer Guy Goldberg says in an interview with BioTuesdays.com, referring to emerging scientific evidence that the microbiome, or the trillions of intestinal microbes, plays a major role in health and disease.
"There's a paradigm shift of viewing the body not as a single organism but as a collection of organisms, with the microbiome as the habitat within the body," he adds.
In 2005, researchers in Australia won the Nobel Prize for identifying H. pylori bacterium and the role it plays in causing peptic ulcers. Stress and lifestyle were long believed to be the primary cause of intestinal ulcers. Fellow Australian, Prof. Thomas Borody, who is now a member of RedHill's advisory board, developed the first antibiotic treatment for ulcers.
RedHill's two lead drug candidates - RHB-104, for Crohn's disease and multiple sclerosis (MS), and RHB-105, forH. pylori - were built on the success of Prof. Borody's approaches to gastrointestinal tract diseases and infections.
"RHB-104 is the biggest program in our pipeline," Mr. Goldberg says. "To the best of our knowledge, we are the only company with a Phase III combination antibiotic approach for treating Crohn's disease, even though there is a lot of academic research connecting Crohn's to a bacterial infection."
RedHill is currently advancing six clinical programs; two of which are expected to be reviewed by the FDA during 2014, and three that are entering pivotal clinical studies.
In a research report last month, Bioassociate Innovative Consulting reiterated its "buy" rating and price target of $14.91 per RedHill American Depositary Share, "given the multiple milestones approaching and the company being on-track with the clinical programs' timelines."
Crohn's is a severe inflammatory disease in the GI tract. Existing drugs, such as Remicade, treat the inflammatory symptoms of the disease by suppressing the immune system. However, they are widely considered to have a poor safety profile and limited long-term efficacy. "There clearly is a strong unmet medical need for a better alternative for Crohn's patients," Mr. Goldberg contends. The global market to treat Crohn's exceeded $3.5-billion in 2012.
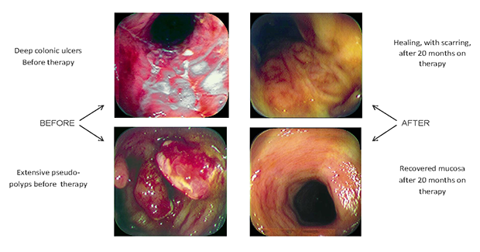
Phase II Study Pictures in Crohn's Patients (Borody et al (2002), Digest Liver Dis 34:29-38)
He explains that RHB-104 is a novel and proprietary combination of three approved antibiotic ingredients - clarithromycin, clofazimine and rifabutin - targeting Mycobacterium Avium Paratuberculosis (MAP). Crohn's patients are believed to have seven times greater likelihood of being MAP-positive than non-Crohn's patients.
The MAP U.S. Study will enroll 240 moderately-to-severely active Crohn's patients at 50 sites in North America and Israel. The primary endpoint is the state of remission at week 26. The study will also examine safety, the maintenance of remission through week 52, efficacy outcome measures in relation to the presence of MAP infection and other secondary endpoints.
If the study is successful, RedHill may submit a new drug application to the FDA in 2015. RHB-104 previously received orphan drug status from the FDA for pediatric use. Mr. Goldberg says discussions are underway with European regulators to begin a second Phase III study with RBH-104 in Europe - the MAP Europe Study.
RedHill also is developing RHB-104 to treat patients with relapsing remitting MS. In June, the company commenced a Phase IIa proof-of-concept trial in Israel. "This is the first time that this type of antibiotic therapy will be tested on MS patients in a Phase II clinical trial," Mr. Goldberg contends.
RedHill's second lead program is RHB-105 in development to treat H. pylori bacteria, which plays an important role in gastritis, peptic ulcers and gastric cancer. Mr. Goldberg points out that H. pylori is increasingly developing resistance to treatment with clarithromycin and metronidazole, and that standard therapy fails in up to 30% to 40% of patients who continue to remain H. pylori-positive.
A Phase IIa study in 2005 by Prof. Borody in Australia demonstrated over 90% eradication of the bacteria in 130 patients who had previously failed standard therapy with clarithromycin. Approximately three millionH. pylori-infected patients are treated annually in the U.S. market, resulting in a potential market estimated at $1-billion to $1.5-billion.
Mr. Goldberg explains that RHB-105 is a novel all-in-one combination of two antibiotics - rifabutin and amoxicillin - and a proton pump inhibitor, omeprazole. RedHill plans to begin a Phase II/3 clinical trial with RHB-105 during the quarter, he says, adding that a successful study could lead to an NDA filing in 2014.

No comments:
Post a Comment